Author:: Josu Galarza
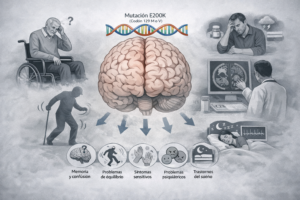
Huntington's disease is a neurodegenerative disorder classified as a rare disease, with an incidence of between 5 and 10 cases per 100,000 inhabitants. Hereditary in nature, its main clinical manifestations are involuntary movements, as well as cognitive and psychiatric alterations. The presence of toxic protein aggregates in the brain, together with its mechanism of propagation, has led it to be considered a prion-type disease.
A recent study—the data from which has not yet been published in its entirety—has demonstrated the ability to slow the progression of the disease, improving patients' independence and well-being. The study, announced in a press release by the Dutch company uniQure (original press release in English here) , uses gene therapy as a tool to silence the gene responsible for brain toxicity in Huntington's patients, thereby delaying their cognitive and motor deterioration. This is vitally important, as the clinical course of this disease can last between 15 and 20 years, during which time patients suffer severe muscle pain due to the onset of persistent involuntary movements.
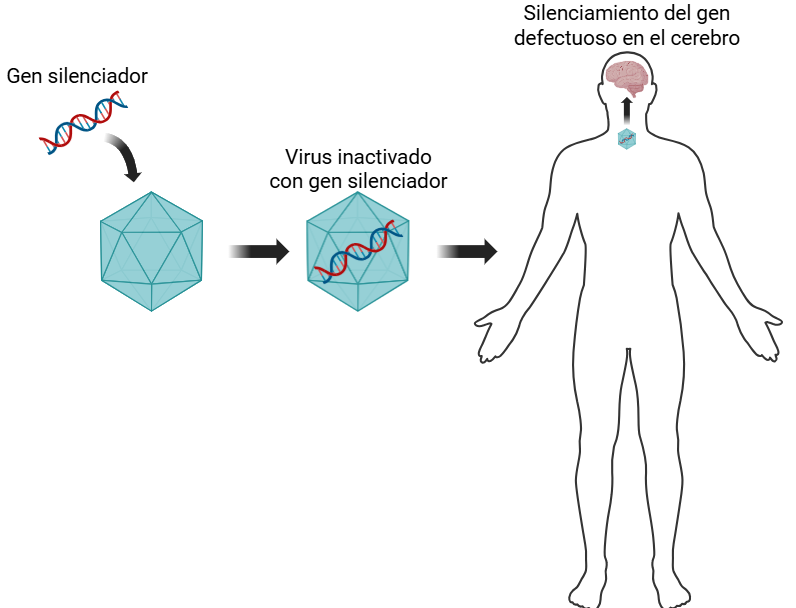
The drug, called AMT-130, is composed of a harmless type of virus called AAV (adeno-associated virus) capable of introducing a fragment of DNA into cells. This DNA sequence is designed to bind to the affected gene and prevent the production of the toxic protein, thus slowing the progression of the disease. The treatment is administered by direct infusion into the central nervous system through catheters in the most affected regions of the brain. The results are promising: the 12 patients who received the highest dose of the drug were observed for 3 years and showed an average reduction of 0.38 points on the Huntington's disease assessment scale, compared to a reduction of 1.52 points in the comparison group, representing a 75% improvement. They also showed less deterioration in other clinical tests and an 8.2% reduction in a cerebrospinal fluid protein called light neurofilament, a marker that reflects neuronal death.
After completing this phase I/II study, uniQure aims to obtain marketing approval over the course of the next year. Approval from health authorities and positive results from this study are crucial for the acceptance of future gene therapies that use similar strategies, opening the door to the study of therapies for other diseases, including prion diseases.