Author: Hasier Eraña
From the laboratory of Sonia Vallabh and Eric Minikel, at the Broad Institute in Boston, comes news which Eric himself reports on his blog (cureffi.org). On 14 April, these researchers achieved a rare milestone in academic research (that which is done outside the pharmaceutical industry), the authorisation of an Investigational New Drug (IND) by the US Food and Drug Administration (FDA, the agency responsible for authorising human trials of new drugs). This is an important step forward on the long and costly road from discovery to development and commercialisation of a new therapy, which in this case is a novel approach to reduce PrPC in the brain using divalent siRNA. Although the researchers themselves recognise that there is still a long way to go to prove the efficacy and safety of this new treatment in patients with prion diseases, they wanted to celebrate the good news in a comprehensive blog post, detailing the current state of development of this potential therapy, and describing the next steps in the process. Below, I summarise the contents of the article published by Eric Minikel on 14 April, the original version of which can be found at cureffi.org.
The journey to a drug candidate
In 2019, Dr Anastasia Khvorova from the University of Massachusetts Medical School visited the Broad Institute to talk about her career path in engineering better short interfering RNA, or siRNA, drugs. She focused on a then very recent milestone: her lab's invention of what they call divalent siRNA.
Until then, antisense oligonucleotides (ASOs) were the only option for reducing a target protein in the brain (they are now in a Phase I trial thanks to a collaboration with Ionis Pharmaceuticals). But we needed another shot at the goal. The publication on divalent siRNA from Anastasia showed a very deep and long-lasting knockdown of a target gene (HTT), and we thought that, if we could do this with our gene, PRNP, it would be really promising.
His team quickly screened siRNA sequences against mouse and human genes, and filed a patent on several of them [WO2021173984]. Combining preliminary data from his lab on siRNA sequences and preliminary data from our lab on new mouse models of human PrP and an assay to quantify PrP depletion in the brain, we applied for a grant from the National Institutes of Health (NIH) to support drug development. Surprisingly, we got it.
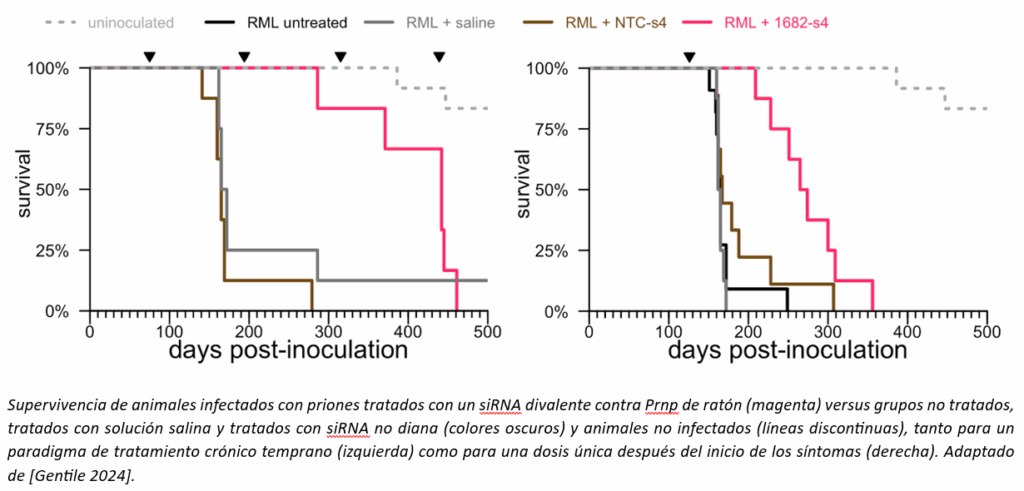
We tested the siRNA molecules sent from the University of Massachusetts, first in cells and then in mice, to find the best molecule. The best siRNA we could find against mouse Prnp was not exceptionally potent; it only reduced PrP in the whole mouse brain by about 50%. But that was enough to ask: does PrP reduction with a divalent siRNA improve survival, as we had seen with ASOs? These are long-term experiments, where a group of animals must be infected with prions and, months later, receive the drug at a very specific time. The experiment worked: treatment with divalent siRNA, even a single dose just after the onset of symptoms, made a big difference to the lifespan of prion-infected animals.
Meanwhile, by autumn 2022, we had settled on a single divalent siRNA compound against human PRNP that, according to all the experiments we had been able to conduct, was the best of the bunch. We saw a depth of PrP reduction we had never seen before, with months of durability, and it appeared to be well tolerated. We desperately wanted this to be another horse in the race to treat prion disease. The choices were clear: either we went about developing this drug ourselves, or we accepted the stark reality that all this work would be "just a study in mice", yet another scientific paper that says "this looks promising" but then never makes it into the lives of any human beings.
At about the same time, we learned of a new NIH funding mechanism, a programme focused precisely on our situation: you have promising data in mice, but a first dose in humans is years of work and millions of dollars away. This programme seemed to be our only chance, but there was no doubt: even with NIH financial support to make the task feasible, this was going to be a very, very big effort for us.
A scientific parenthesis: How does divalent siRNA work?
siRNA stands for short interfering RNA. This type of drug is composed of the same building blocks as DNA and RNA (A, C, G and T or U) and acts through the mechanism of RNA interference (RNAi). RNAi was discovered in 1998 and earned its discoverers the Nobel Prize in Physiology or Medicine in 2006. The siRNA drug is designed to specifically bind to a target RNA molecule in the cell, in this case the one that gives rise to PrP. The result is less target RNA and therefore less of the protein it encodes. Thus, an siRNA is a way to reduce the amount of a specific disease-causing protein. The first siRNA drug, Patissiran for transthyretin amyloidosis, received FDA approval in 2018.
An siRNA is similar to an ASO in that it is a drug designed to bind to and degrade a target RNA. Like an ASO, in humans this siRNA would be administered into the cerebrospinal fluid (CSF) via an intrathecal injection, in other words, a lumbar puncture (LP). An siRNA is different from an ASO in that it is a double-stranded (as opposed to single-stranded) oligonucleotide, uses different chemical modification patterns to stabilise it, and acts through a different cellular pathway (RISC as opposed to H1 RNAase).
As of last year, there were six FDA-approved siRNA drugs, and dozens more in clinical trials. Several different pharmaceutical companies are developing siRNAs with: different chemical modifications, different conjugates to promote uptake in different tissues of the body, and different sequences for different targets in different disease indications.
A divalent siRNA is a special type developed by Anastasia Khvorova's lab. It consists of two copies of the same double-stranded siRNA, linked together. Empirically, studies in mice conducted by Khvorova's lab when they were developing this technology found that single (monovalent) siRNAs with their particular chemistry were simply not taken up and retained in the brain. When they joined two of them together to form a divalent molecule, suddenly a large amount of drug was retained in the brain, was taken up by the cells and was pharmacologically active.
Climbing the IND (Investigational New Drug) mountain
In the US, and really in every country on Earth, the steps necessary to go from "looks good on a mouse" to permission to dose a person are not only time-consuming and expensive, but also highly specialised studies. There is a whole industry that, if you don't work in drug development, you've never seen or thought about, all run by companies whose names you don't know. Chemical synthesis, purification, quality testing and drug packaging (Chemistry, Manufacturing and Controls, CMC, in industry jargon) must be done under Good Manufacturing Practice (GMP), which is 10 times more expensive than making a drug for use in mice. First, all GMP steps are followed to manufacture a drug substance (DS), a white powder, then a complete additional process is followed to formulate that powder into a drug product (DP), which could be a pill or, in our case, a vial of sterile injectable liquid. Toxicology studies must be conducted under Good Laboratory Practice (GLP), with tens or hundreds of animals, each producing hundreds or even thousands of measurements and readings to determine if the drug is toxic. The animals' blood and/or tissues are analysed using a GLP-validated pharmacokinetic (PK) assay to confirm how much drug is actually present and to ensure that they were dosed correctly. There are drug-drug interaction (DDI) studies, to see if patients taking any of the hundreds of approved drugs should be prohibited from taking their drug. There are genotoxicity studies, to determine whether the drug can mutate DNA and cause cancer or birth defects.
The point of all this is to bring it to yet another acronym: the IND, or Investigational New Drug. An IND application is the package of data from all previous studies that a drug developer or sponsor submits to the FDA for permission to dose a human being with a new drug for the first time.
Since we submitted our proposal to the NIH in October 2022, we knew it would be the better part of a year before funding could be awarded, plus probably some delay after that before contracts could be established and work could begin in earnest. Wanting to move forward as quickly as possible, we started manufacturing the drug immediately using Prion Alliance funding. In June 2023, Sonia and I had our first Pre-IND meeting with the FDA, where we were able to ask questions about what would be required for our IND given the particularities of our specific drug and disease. With the FDA's advice in hand, we worked with some extremely smart, dedicated and passionate donors to raise funds for some of the other more limiting steps, particularly the first round of toxicology studies and the development of a pharmacokinetic (PK) assay, the method for quantifying the drug in blood or tissue.
Our financial resources were still quite limited, and we felt that speed was of the essence, so we made some difficult compromises. We opted to conduct a single-dose toxicology study in animals, which, the FDA made very clear to us, would only allow us to conduct a single-dose trial in patients. We made this decision for several reasons. We could only afford a single-dose study, and a single-dose study would be faster. Our data in mice showed that a single dose could have activity lasting 4-6 months, and a single dose at a symptomatic stage of the disease prolonged survival in prion-infected mice by 3.5 months. Therefore, considering that many patients with prion disease live only a few months from diagnosis, we felt that even a single dose had the potential to be clinically relevant. Finally, a single-dose toxicology study presented less risk. As a rule of thumb, toxicology studies are already typically conducted with several times the equivalent of the dose you expect to use in humans. Add to this a monthly administration for 9 months (as is typical in chronic toxicity studies), and the animals receive a very large amount of drug, which greatly increases the likelihood of toxic effects that would prevent progressing to clinical trials.
In an incredible stroke of good fortune, our grant proposal to the NIH was finally funded. This gave us a path forward to conduct all the remaining studies necessary to obtain an IND. This process required 2.5 years of intense work, carried out jointly and largely led by Project Manager Alissa Coffey.
By early 2025, all the IND enabling studies were completed, and what remained was to write the IND itself. Although we had consultants, finding templates and examples to work from was a major challenge. Industry conducts its INDs to an industry standard, which is even more than what is required for a researcher-initiated IND. Academics who have submitted INDs, and there are not too many examples to begin with, have generally not done so for novel drug modalities, such as divalent siRNA. And INDs, whether industrial or academic, that are available, are very hard to come by. But very generously, some people shared their INDs with me, so I had some kind of starting point to work from. On 5 February 2025, Alissa and I sat down together in my office and clicked send.
What our open IND means
When the FDA approves an IND, it not only approves a drug, it approves a specific batch of drug for a specific patient population, treated according to a specific clinical trial protocol. Every detail must be specified. In our IND, we submitted two trial protocols: one to treat symptomatic patients already diagnosed with prion disease, and one to treat pre-symptomatic patients at risk of prion disease. On 14 March, the FDA responded that "we can proceed" in symptomatic patients, while we are in "partial clinical hold" for presymptomatic patients. That means they want to see more data before allowing us to dose presymptomatic people, and they have sent us a detailed letter outlining everything we will need to do to unblock presymptomatic dosing.
But in the meantime, we have an open IND: the FDA is allowing us to start a clinical trial in symptomatic patients with prion disease. I wish that meant we could start dosing people tomorrow, but the reality is more complicated. First, we don't yet have the funding to conduct a clinical trial. The NIH has a programme for this as well, and after successfully passing Stage 1 for this funding mechanism, and within days of receiving a response from the FDA, we submitted our Stage 2 proposal. Whether and when this will be funded remains an unknown. The earliest it could happen is within a couple of months. But it could also be several months, or never.
Suppose someone wrote us a cheque today for the full cost of running a trial. Even then, it would take time to get it up and running. A clinical trial is an extremely complex undertaking. In addition, if the NIH funds the trial, it will determine various details of how the trial will be conducted, and once ethics approval is obtained, there will still be a long list of tasks to be completed.
I am more anxious than anyone to know the timeline for when we will start dosing people, but there are big unknowns at the moment, including that we cannot yet rule out that the answer is "never" if we cannot find the funding. When I have spoken to people with experience in first-in-human trials, most have told me that, if funding comes soon, it would be possible to administer the drug to a first patient by the end of 2025.
What to expect when we are ready to administer the drug to patients
If funding allows, sometime in the next few months we may be able to announce that a clinical trial is open and begin accepting patients. Here are some things to keep in mind when that happens:
First, a clinical trial is research, not treatment. We are conducting a clinical trial precisely because we do not yet know if the drug is safe or effective. All drugs cause some kind of adverse effect, and most drugs that enter trials never benefit anyone. By definition, there is no human data yet to suggest that divalent siRNA will benefit anyone with prion disease. Anyone who volunteers for a trial, this or any other trial, should do so with the expectation of contributing to the research, not benefiting personally.
Second, we are currently limited to administering a single dose of the drug. This is because, to date, we only have single-dose toxicology data in animals. We are working on launching chronic toxicology studies, but we do not yet know the timeline and outcome. Therefore, at this stage, we cannot at all promise anyone in the trial that there will ever be a chance to receive another dose.
Third, we are not a commercial sponsor, and while we are fortunate to have come this far, it is highly unlikely that we will be able to find funding as an academic laboratory to conduct the kind of larger clinical trials that would ultimately lead to approval of a drug. Therefore, we cannot promise a potential future in which this drug will be available.
Fourth, again due to limited resources, we have not yet explored the possibility of administering the drug to patients outside the United States. We would love to see divalent siRNA have a global future for patients everywhere. For today, the FDA is the regulatory agency we know best and have the best access to as Americans, so that's where we went to discuss how to get approval for a first-in-human dose. Meanwhile, the NIH funding that got us here was geared to meet US regulatory requirements, and the NIH funding we have applied for would only support one trial in the US.
For all the above points, remember that it is not that we don't want to do more. Our choice so far has been between doing nothing and doing something, and at every juncture, we have chosen to do something. Now let's talk about what we believe we can achieve by conducting a divalent siRNA clinical trial. The primary goal of a trial will be to obtain preliminary data on whether the drug is safe at the doses tested. Secondly, the trial will assess whether the drug was able to achieve its goal, i.e. to reduce PrP in the brain, at those same doses. No divalent siRNA for any disease has ever been in humans before. And only 1 drug designed to reduce PrP, Ionis' ASO ION717, has been in humans before. We believe that a small first trial of divalent siRNA in prion disease has the potential to teach us about how divalent siRNA works in the human body (its safety, potency, duration of action), as well as about PrP reduction in patients with prion disease. At the same time, a trial will also teach us about prion disease and how to conduct clinical trials in this disease. For example, what kind of patients we can recruit, at what stage of the disease, how fast they progress on various measures, and perhaps what are the kinetics of PrP reduction in this population. We learn a lot from every trial that is done in our disease, and we have a lot more to learn.
Ionis' global PrProfile trial of ION717, even while still ongoing, has taught us that our patient community is large and motivated: people showed up in such numbers that the trial had to be suspended for 4 months. There are many, many more lessons to be learned from prion disease trials, which will be incredibly valuable for the next person designing a trial of (hopefully) an even better drug. Learning some of those lessons in the context of an investigator-initiated study is especially valuable, because we, as academics and especially as patient-scientists, can choose to share data to the greatest extent compatible with patient privacy, in order to maximise generalisable knowledge and benefit to our entire research community. Certainly we, the patient community, can and should push companies to share more data publicly, and to some extent they will listen to us, but publicly traded companies also have a legal obligation to do the right thing by their shareholders and, realistically, they will never share as much as we want them to.
Finally, although it is still difficult to predict the outcome, there is a possibility that divalent siRNA could play an important role in the treatment of prion disease, provided that the results of the first human trial are promising. It is worth noting that good results in murine models are relatively common, but it is the data in patients that are really decisive. If the initial results in humans prove to be positive, this could attract the interest of a sponsor with greater resources than ours, who may be able to push it through development into an approved drug. Let's not forget that the ultimate goal of all our research is to achieve a safe and effective drug for both treatment and prevention of prion disease.
What happens if two trials are recruiting simultaneously?
At this point, Ionis' PrProfile trial has closed enrolment, and Ionis has not yet announced an upcoming ION717 trial. But there may be overlap between a future ION717 trial and a future divalent siRNA trial. Why do we need more than one drug in clinical development? Because we want to maximise the chance of ultimately getting one or more safe and effective drugs. We also want to maximise the research knowledge we gain along the way. Most drugs fail, and even when they succeed, it is rare that the first drug in a previously untreatable disease is the only drug anyone will ever need. It usually takes many different efforts to finally turn a uniformly fatal disease into a manageable or preventable condition. We need multiple goal opportunities.
Is it OK to have more than one drug in clinical development? Yes. 500 patients are diagnosed with prion disease each year in the US alone. There are probably a few thousand cases diagnosed each year worldwide. The Ionis PrProfile trial enrolled only 56 patients worldwide. The dramatically fast enrolment of that trial shows that patients exist and are highly motivated. Unfortunately, we have more than enough patients to enrol multiple drug trials at the same time. And unfortunately, most patients die very quickly, which means that enrolling patients this year does not "exhaust" the patient population for next year.
If patients are eligible for more than one trial, how will they choose which one to enrol in? That's a conversation they should have with their doctor; I can't advise them on that. I have previously shared some of what I think are the biggest caveats about our drug. When a trial is finally recruiting, there will be a consent form that will give you a lot more to think about. Above all, remember that this is research. At the time of writing, there is no human data to suggest that any drug is effective against prion disease.
Transparency
As patient-scientists, we want to maximise prion disease research, and that means a commitment to share our knowledge and data as much as possible. If you've been following this blog, you know we've been harping on this for years, but given that we're now at a new stage where human trials could be close, here's a new preview of that commitment.
NOTE: This section shares technical details about the potential drug. Although they are not reproduced here for ease of reading this adapted version, all information is publicly available in the original article at cureffi.org.
Conclusions
We developed a new prion disease drug candidate in our lab, obtained FDA permission to administer the drug to human patients and are now seeking funding to launch a clinical trial. The trial has not yet been launched. Don't write to me asking how you can get the drug: there is no way yet. We are working hard on this. If you can help us find the funding, let us know. When we get to a trial, it will still be research, not treatment, and no one should participate with the expectation that it will help them. If you want to keep up to date with new developments, follow this blog and, while you're at it, join our mailing list and consider joining PrionRegistry if you want to volunteer for research. Stay tuned.