Author: Nuno Anjo
Sporadic Creutzfeldt-Jakob disease (sCJD) is a rare, fatal and rapidly progressive neurodegenerative disease for which there are still no effective treatments. It is characterised by the misfolding of the normal prion protein (PrPC) in an anomalous form called PrPSc or Prion, which accumulates in the brain, causing neuronal damage and spongiform degeneration. Although it accounts for 80 % of human prion diseases and its aetiology is poorly understood, this study opens up new possibilities for the development of treatments. Researchers from China conducted a groundbreaking study using an integrative multi-omics approach to identify potential therapeutic targets/targets for sCJD. The integrative approach combines data on gene expression, DNA methylation and protein levels in blood and brain tissues to identify genes and pathways relevant to SCJD.
The study analysed genetic data from 4,110 sCJD patients and 13,569 controls, obtained from brain and blood samples in genome-wide association studies (GWAS). The researchers used multi-omics analysis to identify genomic regions (QTLs) that influence gene expression (eQTLs), DNA methylation (mQTLs) and protein levels (pQTLs), providing clues to the molecular mechanisms involved. QTLs are regions of the genome that influence these molecular features and can provide clues about how genes are involved in diseases. In addition, they used single-cell gene expression data (sc-eQTLs) to analyse how these genetic variants affect different types of brain cells. This allowed the researchers to link molecular changes in genes to their impact on specific tissues and cells.
To confirm that the identified genes do indeed play a causal role in sCJD, they employed a statistical method called Mendelian randomisation (MR). This approach uses genetic variants as tools to establish causal relationships between genes and disease, minimising bias from external factors and avoiding confounding caused by indirect relationships. In addition, they conducted colocalisation analyses to determine whether the same genetic variants were involved in both molecular traits and sCJD risk. This approach allowed them to identify genes whose influence on the disease was more robust and less susceptible to interpretative errors.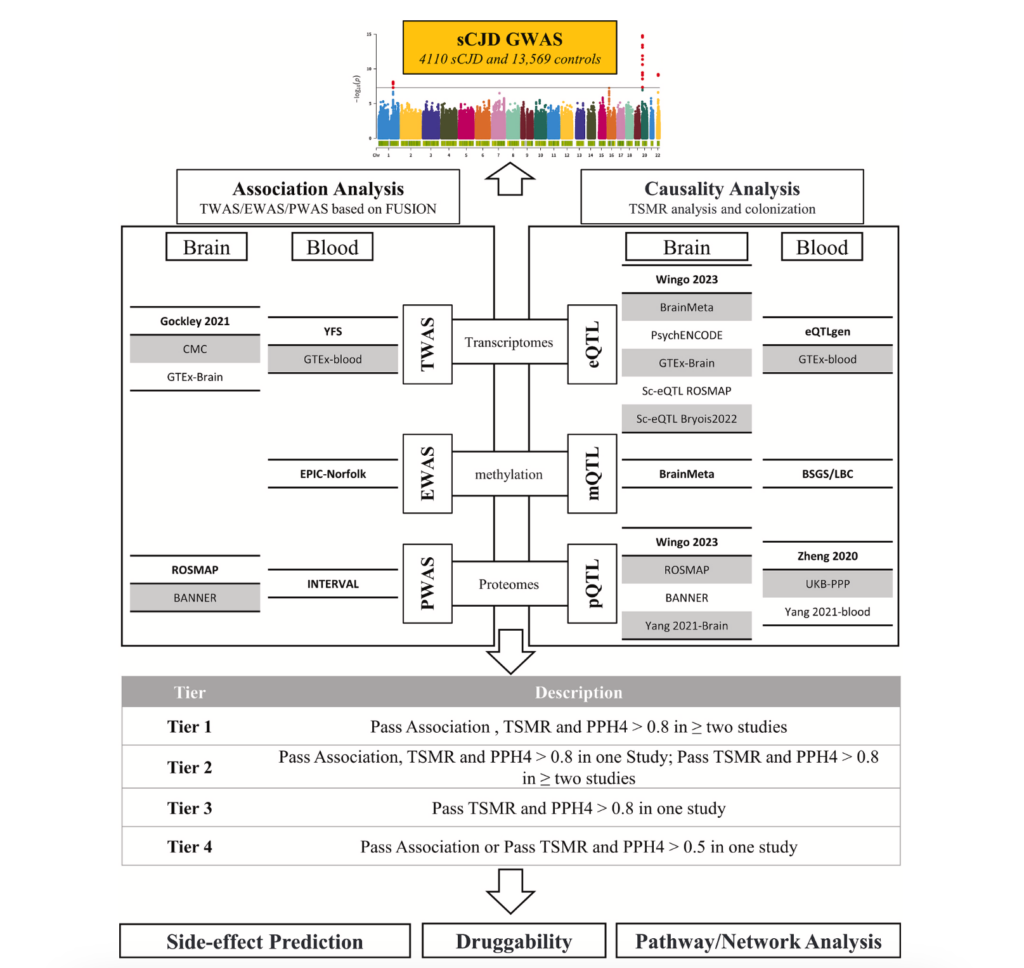
The analysis revealed 23 genes associated with sCJD, including five with the highest level of evidence: STX6, XYLT2, PDIA4, FUCA2 and KIAA1614. The STX6 gene proved to be the most relevant, as its increased expression in brain regions such as the hippocampus and cortex, which are often affected in the disease, was associated with an increased risk of sCJD. STX6 encodes a protein involved in vesicle transport and neuronal communication, key processes that are altered in the disease. Another key gene identified was XYLT2, which encodes an enzyme involved in the synthesis of sulphated proteoglycans, molecules that interact with misfolded prion proteins. This gene could be an interesting therapeutic target, as researchers suggest that drugs such as carboplatin and gemcitabine, currently used in cancer treatments, could be repurposed to treat sCJD. However, further studies are needed to assess their safety and efficacy in this setting.
The proteins encoded in these genes are linked to essential processes such as protein folding, neuronal communication and cellular stress response. For example, PDIA4, another of the identified genes, encodes an endoplasmic reticulum protein involved in proper protein folding. The findings suggest that defects in these processes may contribute to the development of the disease, either by loss of normal functions or by the acquisition of toxic properties in the affected proteins.
This work pioneered the use of multi-omics approaches to investigate prion diseases. The researchers not only identified potential therapeutic targets, but also provided a basis for better understanding the underlying biological mechanisms of sCJD. The results underscore the importance of integrating multiple types of biological data to gain a more complete picture of complex diseases. In addition, the possibility of repurposing approved drugs accelerates the path to potential therapies, although rigorous clinical trials will be needed to validate these proposals. This study represents an important step in sCJD research, identifying potential therapeutic targets and paving the way towards targeted treatments, providing new hope for patients and their families.
See the original article here (in English).