Author: Hasier Eraña
A team of American researchers has published a revolutionary gene editing technique (a technique that allows changes to be made to the genetic material of cells) in the prestigious journal Nature. This technique could change the way we approach the treatment of various genetic or familial diseases with low prevalence, also known as rare diseases, of which up to 7,000 different diseases have currently been described. The new method, called PERT (prime editing-directed editing to read through premature termination codons), has been shown to be capable of restoring protein function in diseases as diverse as cystic fibrosis, Tay-Sachs disease and Hurler syndrome using exactly the same treatment. This ability to treat multiple familial diseases with different causes with a single therapeutic composition represents a radical departure from current precision medicine strategies.
What does this new technique involve?
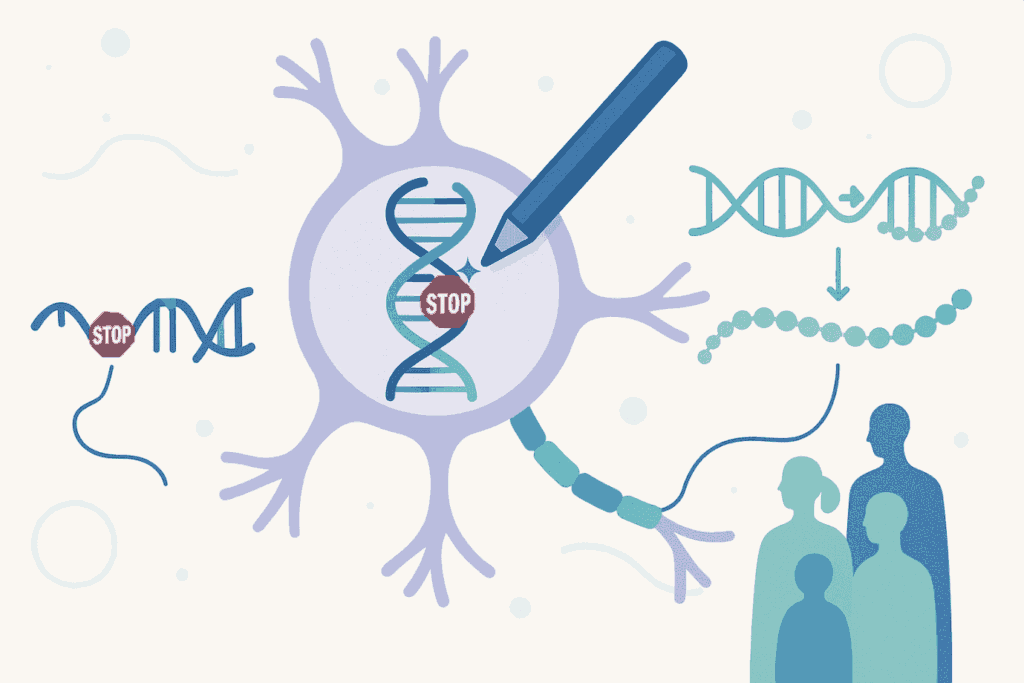
Approximately one quarter of genetic or familial diseases are caused by ‘nonsense mutations’ in a particular gene. Genes provide the instructions for the production of proteins in cells. Nonsense mutations introduce premature stop signals into the genetic instructions, causing cells to stop producing a protein before it is complete. The result is a truncated, inactive protein that cannot perform its function.
The PERT strategy uses an advanced gene editing technology called ‘prime editing’ to permanently convert a redundant transfer RNA (tRNA) gene in the genome into an optimised suppressor tRNA. tRNAs are essential molecules that help translate genetic information into proteins; in other words, they are transporters that link genetic instructions with the components needed to make proteins. Suppressor tRNAs are special versions of these transporters, capable of ‘ignoring’ these premature stop signals, allowing the cell to complete the manufacture of the functional protein, despite the mutation or error in its genetic instructions.
The most innovative aspect of PERT is that, with a single genetic modification administered once (aimed at modifying tRNA), patients with nonsense mutations in completely different genes can be treated. In other words, the same treatment could theoretically benefit people with cystic fibrosis, Duchenne muscular dystrophy, phenylketonuria or Stargardt's disease, among many others, provided that their disease is caused by this specific type of mutation.
What results has the study shown?
Researchers have conducted an exhaustive series of experiments demonstrating the efficacy of PERT in cell models and animal models:
In human cell models of lysosomal diseases (Batten disease, Tay-Sachs disease, and Niemann-Pick type C disease), the treatment restored between 20% and 70% of the normal activity of the proteins involved. These levels are well above the therapeutic threshold required to significantly improve these diseases,
In mice with Hurler syndrome, a severe lysosomal storage disease, PERT restored approximately 6% of the deficient protein activity, resulting in almost complete rescue of all disease manifestations.
The researchers also tested PERT against more than 14,700 pathogenic nonsense mutations recorded in clinical databases, observing that the technique was able to read through the vast majority of them.
Crucially, exhaustive safety analyses detected no reading through natural stop codons (which must function correctly, as they indicate when protein production should end), no alterations in the cellular transcriptome or proteome, and no off-target editing. The treated mice also showed no signs of toxicity due to the treatment during the 15-week follow-up period.
What implications could this have for prion diseases and other neurodegenerative diseases?
While it is important to note that very few prion diseases or other neurodegenerative diseases are caused by nonsense mutations (since most are due to missense mutations or other alterations), this work represents an important conceptual advance for the field of gene therapy and rare diseases in general.
The prime editing technology employed by PERT is extremely versatile and precise, and could be adapted in the future to address other types of genetic mutations. In fact, several research groups, including our laboratory, are actively exploring the use of prime editing and other similar gene editing technologies to develop therapies specifically targeting mutations that cause familial prion diseases.
Furthermore, the concept of developing ‘disease-independent’ therapies that can treat multiple patients with different pathologies using a single composition is particularly relevant for rare diseases such as prion diseases, where the limited number of patients makes it difficult to develop specific therapies for each mutation.
In summary
This study represents an important milestone in the field of precision medicine by demonstrating that it is possible to develop gene therapies that do not need to be designed individually for each mutation, but can benefit patients with multiple different diseases. Although this is still preclinical research and years of additional studies will be necessary before it can be applied in humans, PERT opens the door to a new therapeutic paradigm in which a single drug could transform the treatment of hundreds of genetic diseases, potentially benefiting hundreds of thousands of patients worldwide.
Link to original article.